
Research Group "Microbial Biotechnology"
Departament of Genetics and Microbiology
| Home | Group members | Research | Publications |
 | Marine
environments show a huge microbial and genetic diversity. The research
activity of our group is mainly focused on the study of bacterial
biodiversity and on the characterization of enzymatic activities with
biotechnological interest. We also aim at understanding the
physiological relevance of the enzymes detected. Several bacteria studied by our group have been isolated from the microbiota of the marine plant Posidonia oceanica shown in the picture (Paula Sánchez López y Antonio Sánchez Amat). |
Marinomonas mediterranea is an example of Gram negative marine bacterium that has shown to be a source of several enzymes of biotechnological and scientific relevance.
| CRISPR-Cas systems enable adaptive immunity in prokaryotes against invading genetic elements. The type III-B system of M. mediterranea
shows a Cas1 protein fused to a retrotranscriptase domain. It has been
shown that it is able to acquire spacers from RNA. This system is
also able to use spacers present in other CRISPR arrays to provide an
additional layer of protection to phages that might escape the other
system. s. These studies have been carried out in collaboration with the groups of Dr.
Andrew Z. Fire (Stanford University, USA) Dr. Alan Lambowitz (University of Austin at Texas) and Dr. Peter C. Fineran
(University of Otago, Dunedin, New Zealand) | 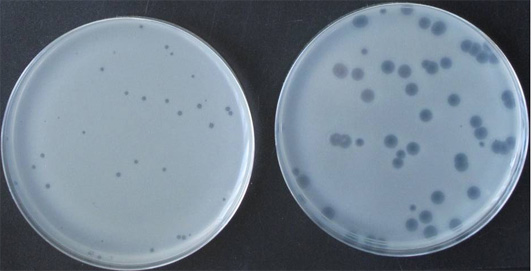 Plaques of the Marinomonas phage CPG1g on different CRISPR-Cas mutants of M. mediterranea |
| In M. mediterranea it has been detected a novel L-lysine epsilon oxidase (EC 1.4.3.20). This
enzyme, named LodA, generates hydrogen
peroxide what confers to it antimicrobial properties. |  Antibiogram of E. coli. The antimicrobial activity of disks containing LodA- marinocine (M) is inhibited by catalase (disk C) but not by the control with buffer (disk B). |
| LodA,
is a quinoprotein containing as cofactor cysteine tryptophil quinone
(CTQ) which is generated by post-translational modification. It is the
first oxidase described with this cofactor. In collaboration with Dr.
Victor L. Davidson (University of Central Florida) we are studying the
generation of CTQ LodA-like proteins are widely distributed in microbial genomes. Some of them show other enzymatic activities such as glycine oxidase activity. | 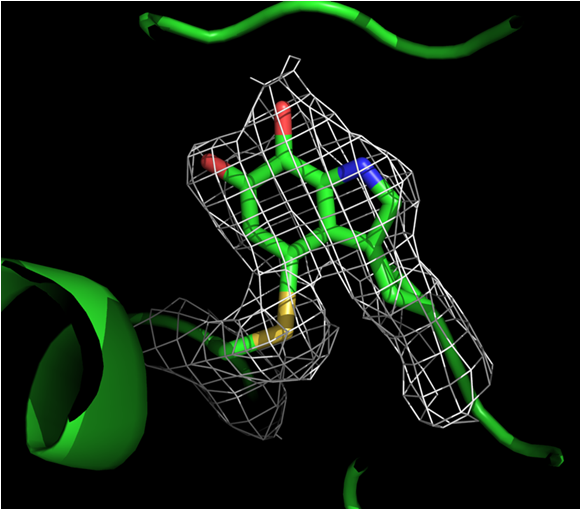 Structure of CTQ cofactor of LodA |
| M. mediterranea expresses
two different polyphenol oxidases (PPOs). The first one is a
multicopper oxidase able to oxidize subtrates characteristics of both
laccases and tyrosinases. The second PPO is a tyrosinase responsible
for melanin synthesis. | M. mediterranea culture in a chemically defined medium containing L-tyrosine. |
